Section 4: Sample Selected-Response Questions Physical Science 6–12 (237)
Expand All Answers | Collapse All Answers
This section presents some sample exam questions for you to review as part of your preparation for the exam. To demonstrate how each competency may be assessed, sample questions are accompanied by the competency that they measure. While studying, you may wish to read the competency before and after you consider each sample question. Please note that the competency statements do not appear on the actual exam.
For each sample exam question, there is a correct answer and a rationale for each answer option. The sample questions are included to illustrate the formats and types of questions you will see on the exam; however, your performance on the sample questions should not be viewed as a predictor of your performance on the actual exam.
The following reference materials will be available to you during the exam:
Domain I—Scientific Inquiry and Processes
Competency 001—The teacher understands how to select and manage learning activities to ensure the safety of all students and the correct use and care of organisms, natural resources, materials, equipment and technologies.
1. Which of the following is safety equipment that can be found in a high school chemistry lab?
- Bunsen burner
- Eyewash station
- Barometer
- Glass mercury thermometer
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because an eyewash station is used to flush the eyes when liquids have been splashed or sprayed into a person's eyes. Option A is incorrect because a Bunsen burner is used to heat some materials in the lab and must be used with care. Option C is incorrect because a barometer is used to measure atmospheric pressure. Option D is incorrect because a glass mercury thermometer can pose a significant hazard due to possible broken glass and mercury exposure.
2. Which THREE of the following are units in the International System of Units (SI)?
- Kelvin
- Pound
- Kilogram
- Mole
- Enter to expand or collapse answer.Answer expanded
- Options A, C, and D are correct because they are each units in the International System of Units (SI). The kelvin is the SI unit of absolute temperature. The kilogram is an SI unit of mass. The mole is an SI unit that is equal to 6.02 times 10 to the power of 23 representative particles. For example, one mole of Na contains 6.02 times 10 to the power of 23 atoms and one mole of O2 contains 6.02 times 10 to the power of 23 molecules. Option B is incorrect because a pound is not an SI unit but is a unit of measurement in a number of other systems of measurement.
Competency 002—The teacher understands the nature of science, the process of scientific inquiry and the unifying concepts that are common to all sciences.
3. Which of the following is a scientific inference?
- Data suggests that Mars once had liquid water
- Repeated measurements of a quantity will reduce random error
- Electrical equipment should be grounded
- A measurement has three significant figures
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because it is a reasonable conclusion drawn from data, which defines a scientific inference. Option B is incorrect because it is a mathematical statement of fact. Option C is incorrect because it describes a safety practice. Option D is incorrect because it is an observation.
Competency 003—The teacher understands the history of science, how science impacts the daily lives of students and how science interacts with and influences personal and societal decisions.
4. Of the following, which contributes the most to water pollution in streams near mountains?
- Nuclear power plants
- Mine drainage
- Carbon dioxide emissions from gas-powered automobiles
- Oil-well drilling
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because acid and metal ion mine drainage from abandoned coal mines has a significant impact on many streams in mountainous coal-mining regions. Option A is incorrect because although nuclear power plants can contribute to thermal pollution, plants are typically located near rivers or oceans, not in mountain regions near streams. Radioactive emissions are not common and are not the major source of water pollution in mountain streams. Option C is incorrect because carbon dioxide emissions can lead to a minor amount of dissolved carbon dioxide (carbonic acid), but the level is not considered significant. Option D is incorrect because oil well drilling is not typically done in areas that could affect streams in mountainous regions.
5. Historically, the Bohr model was successful in explaining which of the following?
- The emission spectrum of the hydrogen atom
- The red shift in the spectrum of light from distant stars
- The black-body radiation spectrum
- The photoelectric effect
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the primary success of the Bohr model was its derivation of the Rydberg formula for the spectral emission lines of hydrogen. Option B is incorrect because the Bohr model is not germane to the red shift of light coming from distant stars, which is explained by the Doppler effect. Option C is incorrect because the Bohr model is not germane to the spectrum of black-body radiation, which is explained by Planck's radiation formula. Option D is incorrect because the Bohr model is not germane to the photoelectric effect, which is explained by Einstein's photoelectric equation.
Domain II—Physics
Competency 004—The teacher understands the description of motion in one and two dimensions.
6. A ball of mass m is thrown horizontally with an initial speed upsilon subscript 0 from the top of a building that is height h above level ground. In the absence of air resistance, how much time will elapse before the ball strikes the ground?
- upsilon subscript 0 over g
- h over upsilon subscript 0
- the square root of 2gh
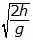
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because it properly recognizes that the horizontal and vertical motions of the ball are independent of each other and then uses the equations of straight-line motion applied to the vertical motion of the ball to calculate the time that elapses before the ball strikes the ground. The kinematical equations of motion give h equals 1 half gt squared. Thus, solving for the time t gives t2squared = 2h over g, or t =
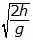 . Option A is incorrect because it assumes that upsilon subscript 0 = gt, which is not true. Option B is incorrect because it ignores gravity and assumes that the vertical component of the speed is constant and equal in value to upsilon subscript 0, which is not true. Option C is incorrect because it is equal to the vertical component of the speed of the ball just before it strikes the ground and not the time it takes to fall.
. Option A is incorrect because it assumes that upsilon subscript 0 = gt, which is not true. Option B is incorrect because it ignores gravity and assumes that the vertical component of the speed is constant and equal in value to upsilon subscript 0, which is not true. Option C is incorrect because it is equal to the vertical component of the speed of the ball just before it strikes the ground and not the time it takes to fall.
7. Two satellites are each in a circular orbit around Earth at a distance R and 2R, respectively, from Earth's center. If the satellite at distance R has an orbital speed of upsilon, the satellite at distance 2R must have an orbital speed equal to
- 2 upsilon
- the square root of 2 upsilon
- upsilon
- upsilon over square root of 2
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because it properly applies the equation for an object in circular motion in Earth's gravitational field to compute the orbital speed. The equation for the orbital speed is upsilon squared = GM over R, where G is the universal gravitational constant, M is the mass of the Earth, and R is the distance from Earth's center. When the orbital radius is doubled, the orbital speed decreases by a factor of 1 over square root of 2. Option A is incorrect because it assumes that the orbital speed is proportional to R, which is not true. Option B is incorrect because it assumes that the orbital speed is proportional to the square root of R, which is not true. Option C is incorrect because it assumes that the orbital speed does not depend on the radius of the orbit, which is not true.
Competency 005—The teacher understands the laws of motion.
8. A 50 N force is applied to a 10 kg block that is initially at rest on a rough horizontal surface. If the block accelerates uniformly at 2 m/s2 squared, what is the magnitude of the frictional force acting on the object?
- 98 N
- 50 N
- 30 N
- 20 N
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because it properly assumes that the net force acting on the block is equal to the applied force minus the frictional force, and then it applies Newton's second law of motion to obtain the equation 50 N − f = 10 kg × 2 m/s2 squared, which, solving for the frictional force f, gives f = 30 N. Option A is incorrect because it assumes that the frictional force is equal to the weight of the object, or 10 kg × 9.8 m/s2 squared, which is not true. Option B is incorrect because it incorrectly assumes that the frictional force is equal to the applied force of 50 N. Option D is incorrect because it assumes that the frictional force is equal to the mass of the block times its acceleration, or 10 kg × 2 m/s2 squared, which is not true.
Competency 006—The teacher understands the concepts of gravitational and electromagnetic forces in nature.
9. A simple pendulum with period T on Earth is transported to the Moon where the gravitational force is about one-sixth the gravitational force on Earth. The period of the pendulum on the Moon is approximately equal to which of the following?
- 6T
- the square root of 6 T
- T
- T over 6
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because the period of a simple pendulum is equal to
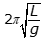 , where L is the length of the pendulum and g is the acceleration due to gravity, and because
, where L is the length of the pendulum and g is the acceleration due to gravity, and because 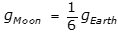 , the period on the Moon is equal to the square root of 6 T, as the following calculation shows:
, the period on the Moon is equal to the square root of 6 T, as the following calculation shows:
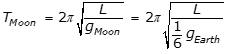 .
.
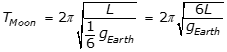 .
.
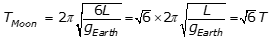 .
.
Option A is incorrect because it assumes that the period of a simple pendulum is proportional to 1 over g instead of 1 over the square root of g, where g is the acceleration due to gravity. Option C is incorrect because it assumes that the period of the pendulum is independent of the acceleration due to gravity g. Option D is incorrect because it assumes that the period of a simple pendulum is proportional to g instead of 1 over the square root of g, where g is the acceleration due to gravity.
10. On the basis of Coulomb's law, which of the following is true about the electrostatic force between two charges?
- It increases in magnitude as the distance between the charges increases
- It is dependent on the masses of the charges
- It can be attractive or repulsive
- It is equal to the magnetic force between the charges
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because according to Coulomb's law, the electrostatic force between two charges depends on the relative signs of the charges and will be attractive when the two charges are oppositely charged and will be repulsive when the two charges are either both positively charged or both negatively charged. Option A is incorrect because according to Coulomb's law, the electrostatic force between two charges is inversely proportional to the square of the distance between the charges and, therefore, decreases in magnitude as the distance between the charges increases. Option B is incorrect because according to Coulomb's law, the electrostatic force between two charges is not dependent on the masses of the charges. Option D is incorrect because Coulomb's law is not concerned with magnetic forces.
Competency 007—The teacher understands applications of electricity and magnetism.
11. A 5 ohm resistor connected in series with a voltage source dissipates 20 W of power. If the source voltage is doubled, the power dissipated by the resistor will be equal to which of the following?
- 80 W
- 40 W
- 20 W
- 5 W
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because Ohm's law gives the equation P = V squared over R for the power P dissipated by a resistor, where R is the resistance of the resistor and V is the voltage across the resistor. Thus, doubling the source voltage quadruples the power dissipated, giving a value of 80 W. Option B is incorrect because it assumes that doubling the source voltage doubles the power dissipated, which is not true. Option C is incorrect because it assumes that doubling the source voltage has no effect on the power dissipated, which is not true. Option D is incorrect because it assumes that doubling the source voltage reduces the power dissipated by a factor of four, which is not true.
12. A circuit consists of a 1.0 ohm resistor and a 4 ohm resistor connected in parallel to a 20.0 volt source. What is the total current in the circuit?
- 4.0 amps
- 5.0 amps
- 15 amps
- 25 amps
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because it is based on the fact that the voltage across each resistor in this parallel circuit is 20.0 volts. Based on Ohm's law (V = IR) the current through the 1.0 ohm resistor is 20 amps and the current through the 4.0 ohm resistor is 5.0 amps. The total current in the circuit is equal to 20 amps + 5.0 amps = 25 amps. Option A is incorrect because it gives the result for a circuit in which the resistors are connected in series. Options B and C are incorrect because they each give a result that is not the current for this circuit.
Competency 008—The teacher understands the conservation of energy and momentum.
13. A railroad boxcar of mass M is moving along a straight horizontal track with speed υ. It collides and couples with a second boxcar of mass 4M that is at rest. What is the kinetic energy of the coupled boxcars immediately after the collision?
- 0
- 1 tenth M upsilon squared
- 1 half M upsilon squared
- 5 over 2 M upsilon squared
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because it properly applies the law of conservation of linear momentum to determine that the correct speed of the coupled boxcars just after the collision is given by the equation M upsilon equals left paren M plus 4M right paren upsilon subscript coupled, which can be solved to give upsilon subscript coupled equals upsilon over 5. It then uses this speed to calculate that the kinetic energy of the coupled boxcars just after the collision is equal to 1 half left paren M plus 4M right paren left paren upsilon over 5 right paren squared equals 1 tenth M upsilon squared. Option A is incorrect because, based on the law of conservation of linear momentum, the coupled boxcars must be moving. Option C is incorrect because it assumes that the kinetic energy is conserved, which is only true of an elastic collision. The coupling indicates that the collision is inelastic. Option D is incorrect because it assumes that the speed of the coupled boxcars is upsilon, which is not true.
14. An object with a mass of 2 kg is accelerated by a force on a frictionless flat surface and is moving in a straight line. The net work done on the object is 20 J. What is the resulting change in the kinetic energy of the object?
- 10 J
- 20 J
- 40 J
- 200 J
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because based on the work-energy principle derived from conservation of energy, the change in kinetic energy is equal to the net work done. The change in kinetic energy is 20 J. Options A, C, and D are incorrect because they are not based on the work-energy principle.
Competency 009—The teacher understands the laws of thermodynamics.
15. The first law of thermodynamics is a statement of which of the following?
- The ideal gas law
- The uncertainty principle
- The law of conservation of energy
- The behavior of the entropy of a system
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because the first law of thermodynamics is simply a statement of the law of conservation of energy. Option A is incorrect because the ideal gas law is a consequence of the kinetic theory of gases, not of the first law of thermodynamics. Option B is incorrect because the uncertainty principle is a consequence of quantum mechanics, not of the first law of thermodynamics. Option D is incorrect because the second law of thermodynamics, not the first law, is a statement about the behavior of the entropy of a system.
16. On a cold night, frost forms on the top of a car but does not form on the underside. The pattern of frost formation indicates that the car is losing heat through what process?
- Radiation
- Convection
- Conduction
- Inertia
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the frost formed on top of the car is the result of the radiative transfer of thermal energy from the car into the air, which cools off the car and allows the formation of frost. Options B and C are incorrect because they are not heat transfer mechanisms relevant to the situation described. Option D is incorrect because inertia is not a heat transfer method.
Competency 010—The teacher understands the characteristics and behavior of waves.
17. A light ray passes from air (n = 1) into glass (n = 1.55). Which of the following is true about the angle of refraction?
- It is equal to the angle of incidence
- It is less than the angle of incidence
- It is equal to the angle of reflection
- It is greater than the angle of reflection
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because by Snell's law, the light ray will be bent toward the normal to the surface when it passes from air into glass, which means that the angle of refraction is less than the angle of incidence. Option A is incorrect because by Snell's law, the light ray will be bent toward the normal to the surface when it passes from air into glass, which means that the angle of refraction is less than, and not equal to, the angle of incidence. Option C is incorrect because by Snell's law, the light ray will be bent toward the normal to the surface when it passes from air into glass, which means that the angle of refraction is less than the angle of incidence. Because the angle of incidence is equal to the angle of reflection, the angle of refraction is less than, and not equal to, the angle of reflection. Option D is incorrect because by Snell's law, the light ray will be bent toward the normal to the surface when it passes from air into glass, which means that the angle of refraction is less than the angle of incidence. Because the angle of incidence is equal to the angle of reflection, the angle of refraction is less than, and not greater than, the angle of reflection.
18. Of the following phenomena, which is characteristic of light but not of sound?
- Diffraction
- Interference
- Polarization
- Dispersion
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because polarization is a phenomenon exhibited only by transverse waves, such as light, and not by longitudinal waves, such as sound. Option A is incorrect because diffraction is a phenomenon exhibited by all waves, including light and sound. Option B is incorrect because interference is a phenomenon exhibited by all waves, including light and sound. Option D is incorrect because dispersion is a phenomenon exhibited by all waves, including light and sound.
Competency 011—The teacher understands the fundamental concepts of quantum physics.
19. According to the Bohr model of the hydrogen atom, the energy En of an electron in the nth energy level of the atom is equal to En = negative 13.6 over n squared eV. What is the energy of the photon emitted when an electron makes a transition from the n = 2 level to the n = 1 level?
- 6.8 eV
- 10.2 eV
- 13.6 eV
- 17.0 eV
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because it properly computes the energy difference as E subscript 2 minus E subscript 1 equals 13.6 over 2 squared eV plus 13.6 over 1 squared eV equals 10.2 eV. Option A is incorrect because it computes the energy difference with 1 over n instead of 1 over n squared. Option C is incorrect because it simply computes the energy of the first level. Option D is incorrect because it makes an error with the algebraic signs of the terms.
20. In the photoelectric effect, light is incident on a metallic surface and photoelectrons are emitted. If f and lambda represent the frequency and wavelength, respectively, of the incident light, and W represents the work function of the metal, which of the following equations correctly gives the maximum kinetic energy E of the emitted photoelectrons?
- E = h lambda
- E = hf
- E = W
- E = hf − W
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because it properly accounts for the fact that the maximum kinetic energy of the emitted photoelectrons depends directly on the frequency of the incident light and that the electrons must have a minimum energy W before they can be emitted from the metal. Option A is incorrect because it assumes that the maximum kinetic energy of the emitted photoelectrons is directly proportional to the wavelength of the incident light, which is not true. Option B is incorrect because it fails to account for the fact that the electrons must have a minimum energy W before they can be emitted from the metal. Option C is incorrect because it assumes that the maximum kinetic energy of the emitted photoelectrons is equal to the work function of the metal, which is not true.
Domain III—Chemistry
Competency 012—The teacher understands the characteristics of matter and atomic structure.
21. Of the following, which is an example of a physical change only?
- Snow sublimating in the Arctic
- An iron nail rusting
- A candle burning
- A lead storage battery recharging
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because snow (H2O) changes state from solid to gas, which is a physical change, and no chemical changes occur. Option B is incorrect because Fe in the iron nail reacts with O2 to form FeO2, which is a chemical change. Option C is incorrect because the combustion reaction of a candlewick with oxygen in the flame is a chemical change. Option D is incorrect because recharging a lead storage battery involves an electrochemical reaction.
22. Based on its position on the periodic table, which of the following elements has the most metallic chemical properties?
- Cs
- Au
- Sb
- Br
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because in the periodic table, metallic chemical properties generally increase going down the columns and decrease going across the rows. Cs is in the lower left corner of the periodic table. Option B is incorrect because Au is much farther to the right than Cs on the periodic table and is a very unreactive metal. Option C is incorrect because Sb is much farther to the right and higher than Cs on the periodic table. Sb is a metalloid. Option D is incorrect because Br is much farther to the right and higher than Rb on the periodic table. Br is a nonmetal.
Competency 013—The teacher understands the properties of gases.
CH4 + 2 O2 produces CO2 + 2 H2O
23. If 44.8 L of O2, measured at 273 K and 1 atm, reacts completely with 2.00 mol of CH4 according to the equation above, what volume of CO2, measured at 273 K and 1 atm, is produced?
- 11.2 L
- 22.4 L
- 44.8 L
- 89.6 L
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because the volume produced is 22.4 L at 298 K and 1 atm. Because 44.8 L of O2 contains about 2 mol of O2, it is the limiting reagent. According to the balanced reaction, 1 mol of CO2 will be produced if the 2 moles of oxygen are consumed. One mol of gas at this temperature and pressure has a volume of about 22.4 L. Option A is incorrect because 11.2 L is half the volume of CO2 that is produced. Option C is incorrect because 44.8 L is twice the volume of CO2 that is produced. Option D is incorrect because 89.6 L is four times the volume of CO2 that is produced.
24. A mixture of gases, 200 L O2, 300 L He, and 500 L N2, each at standard temperature and pressure, are compressed into a 10 L tank. If the total pressure of the compressed gases is 200 atm, which of the following is the partial pressure of O2 in the tank?
- 10 atm
- 20 atm
- 40 atm
- 200 atm
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because the partial pressure of O2 in the tank is the product of the mole fraction of O2 in the mixture and the total pressure. Based on the volumes of the gases in the uncompressed mixture, the mole fraction of O2 is 0.1, therefore the partial pressure of compressed O2 in the tank is 20 atm. Option A is incorrect because that assumes that the mole fraction of O2 in the mixture is 0.05. Option C is incorrect because that assumes that the mole fraction of O2 in the mixture is 0.2. Option D is incorrect because that assumes that the pressure of O2 in the mixture is equivalent to the total pressure of the mixture.
Competency 014—The teacher understands properties and characteristics of ionic and covalent bonds.
25. In which of the following compounds is there both covalent and ionic bonding in the solid state?
- MgCl2
- H2S
- CCl2H2
- CaSO4
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because there is covalent bonding between the S and O atoms that form the polyatomic anion SO4 negative, and there is ionic bonding between the SO4 negative anions and the Ca superscript 2 positive cations in the solid crystal. Option A is incorrect because in MgCl2 there is ionic bonding between Mg superscript 2 positive cations and Cl negative anions. Option B is incorrect because in H2S there is covalent bonding between the H and S atoms. Option C is incorrect because in CCl2H2 there is covalent bonding between the Cl and C atoms and between the H and C atoms.
26. Of the following, which has the highest normal boiling point?
- Ar
- CH4
- NH3
- O2
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because NH3 forms hydrogen bonds in the liquid state and has a normal boiling point of negative 33 degrees C. Option A is incorrect because Ar has weak intermolecular forces and a normal boiling point of negative 186 degrees C. Option B is incorrect because CH4 has weak intermolecular forces and a normal boiling point of negative 164 degrees C. Option D is incorrect because O2 has weak intermolecular forces and a normal boiling point of negative 183 degrees C.
Competency 015—The teacher understands and interprets chemical equations and chemical reactions.
27. Which of the following is the balanced equation for the displacement reaction of potassium with aluminum nitrate?
- 3 K + Al(NO3)3 produces 3 KNO3 + Al
- K + AlNO3 produces KNO3 + Al
- 3 K + AlNO3 produces K3N + AlO3
- K + AlN produces KN + Al
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the reaction of potassium with aluminum nitrate forms KNO3 and Al, and the formula for aluminum nitrate is Al(NO3)3, the formula for potassium nitrate is KNO3 and the equation is balanced with an equal number of each type of atom on the right and left side of the equation (i.e., three K atoms, one Al atom, three N atoms, and nine O atoms). Option B is incorrect because the formula for aluminum nitrate is not correctly represented. Option C is incorrect because the incorrect products are formed, and the formula for aluminum nitrate is not correctly represented. Option D is incorrect because the incorrect products are formed, and the compound formulas are not correctly represented.
C(s) + O2(g) is in equilibrium with CO2(g)
28. Which of the following is the equilibrium constant, KC, for the reaction represented above?
- KC = left bracket CO subscript 2 right bracket over left bracket C right bracket left bracket O subscript 2 right bracket
- KC = left bracket C right bracket left bracket O subscript 2 right bracket over left bracket CO subscript 2 right bracket
- KC = left bracket CO subscript 2 right bracket over left bracket O subscript 2 right bracket
- KC = left bracket O subscript 2 right bracke over left bracket CO subscript 2 right bracket
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because the equilibrium constant in terms of concentration KC is equal to the concentration of each aqueous or gaseous product over the concentration of each aqueous or gaseous reactant, with each raised to the power that is equal to the coefficient of that component in the balanced equation. Because C(s) is a solid, it does not appear in the expression. And because the coefficient for the gaseous product CO2 and the gaseous reactant O2 are both 1, they are each raised to the power of one. Option A is incorrect because [C] should not be included. Option B is incorrect because [C] should not be included; the reactant is in the numerator, and the product is in the denominator. Option D is incorrect because the reactant is in the numerator and the product is in the denominator.
Competency 016—The teacher understands types and properties of solutions.
29. Which of the following is the molar concentration of KNO3 in a 4.00 L solution that contains 50.5 g of KNO3?
- 2.00 M
- 0.500 M
- 0.250 M
- 0.125 M
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because the concentration of KNO3 is 0.125 M, which is calculated as follows: . [KNO subscript 3] equals 50.5 g over 4.00 L times 1 mol KNO subscript 3 over 101 g KNO subscript 3 equals 0.125 M Option A is incorrect because 2.00 M is sixteen times more concentrated than the correct concentration. Option B is incorrect because 0.500 M is four times more concentrated than the correct concentration. Option C is incorrect because 0.250 M is two times more concentrated than the correct concentration.
30. Sugar was dissolved in water at 50°C. When the temperature of the solution was reduced to 30°C, some of the sugar precipitated. Of the following, which best describes the solution after some of the sugar precipitated?
- Saturated
- Supersaturated
- Unsaturated
- Dilute
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the solution is saturated, as evidenced by the precipitation of sugar. Option B is incorrect because supersaturated describes a solution in which more than the maximum amount of solute is dissolved at that temperature and there is no precipitate. Option C is incorrect because unsaturated means that the maximum amount that could dissolve at that temperature has not yet been reached and there is no precipitate. Option D is incorrect because the term "dilute" would indicate that very little sugar is present in the solution, when in fact there is a significant amount of dissolved sugar.
Competency 017—The teacher understands energy transformations that occur in physical and chemical processes.
31. If 5.0 mL of 80.0°C water is mixed with 15.0 mL of 20.0°C water in a thermally insulated container, which of the following will be the temperature of the water once thermal equilibrium is reached?
- 75°C
- 50°C
- 35°C
- 25°C
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because the temperature of the mixture is 35°C at thermal equilibrium, and is calculated as follows: the warmer water loses the amount of heat that the cooler water gains and is found from delta Heat left paren 80 degres C right paren equals negative delta Heat left paren 20 degrees C right paren. Thus, 5 mL times left paren 80 minus T right paren = negative 15 mL times left paren 20 minus T right paren. And solving for T gives T = 35°C. Option A is incorrect because 75°C is much too warm, considering that much less of the warmer water was added. Option B is incorrect because 50°C is too warm, considering that it is not reasonable that the final temperature would be halfway between the two original temperatures given that the volumes of water mixed were not equal. Option D is incorrect because it is too low, based on the correct calculations, although, as a guess, it is more likely than A or B.
Competency 018—The teacher understands nuclear fission, nuclear fusion and nuclear reactions.
32. The half-life of a radioactive isotope X is 12 hours. Starting with a pure 80.0 g sample of the isotope, how much of the isotope will remain in the sample after 48 hours have elapsed?
- 40 g
- 20 g
- 10 g
- 5 g
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because the amount remaining is 5 g. 48 hours is four half-lives. After 12 hours (one half-life), half of the original X atoms will have decayed into another isotope, leaving 40 g of X atoms. Then after another 12 hours, half of the 40 g of X atoms will have decayed, leaving 20 g of X atoms. Then after another 12 hours, half of the 20 g of X atoms will have decayed, leaving 10 g of X atoms. Then after another 12 hours, half of the 10 g of X atoms will have decayed, leaving 5 g of isotope X atoms. Option A is incorrect because 40 g of isotope X atoms would remain after 12 hours. Option B is incorrect because 20 g of isotope X atoms would remain after 24 hours. Option C is incorrect because 10 g of isotope X atoms would remain after 36 hours.
Competency 019—The teacher understands oxidation and reduction reactions.
33. Which of the following is the oxidation number for Cr in K2CrO4?
- positive 1
- positive 4
- positive 6
- positive 8
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because in K2CrO4, the oxidation number for K is positive 1 and the oxidation number for O is negative 2. Because the net charge on the compound is zero, then left paren oxidation number of Cr right paren plus 2 left paren positive 1 right paren plus 4 left paren negative 2 right paren equals 0. Hence, the oxidation number of Cr is positive 6. Option A is incorrect because if the oxidation number was positive 1 for Cr, then the compound would have a net charge of negative 5. But the compound has a net charge of zero. Option B is incorrect because if the oxidation number was positive 4 for Cr, then the compound would have a net charge of negative 2. But the compound has a net charge of zero. Option D is incorrect because if the oxidation number was positive 8 for Cr, then the compound would have a net charge of positive 2. But the compound has a net charge of zero.
34. Which TWO of the following represent reduction processes?
- C U produces C U superscript positive plus E superscript negative
- N A superscript positive plus E superscript negative produces N A
- C U superscript 2 positive plus E superscript negative produces C U superscript positive
- C U superscript positive produces C U superscript 2 positive plus E superscript negative
- Enter to expand or collapse answer.Answer expanded
- Options B and C are correct because they both represent a reduction process in which the oxidation number is reduced as an electron is added. In option B the oxidation number changes from positive 1 to 0. In option C the oxidation number changes from positive 2 to positive 1. Option A is incorrect because it represents an oxidation process in which the oxidation number increases from 0 to positive 1 as an electron is lost. Option D is incorrect because it represents an oxidation process in which the oxidation number increases from positive 1 to positive 2 as an electron is lost.
Competency 020—The teacher understands acids, bases and their reactions.
35. Which of the following is a weak acid?
- HF
- HCl
- HNO3
- R b O H
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because HF is a weak acid that partially dissociates in water with K subscript a equals six times ten to the power of negative 4. Options B and C are incorrect because HCl and HNO3 are strong acids that dissociate almost completely in water. Option D is incorrect because R b O H is a base, not an acid.
36. What is the pH of 0.00006 M HNO subscript 3 left paren aq right paren?
- 4.0
- 4.2
- 5.0
- 6.0
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because pH = 4.2. The pH equals negative log right bracket H superscript positive left bracket. The concentration 0.00006 M can be expressed as 6 times 10 to the power of negative 5 M. Hence, pH equals negative log left paren 6 times 10 to the power of negative 5 right paren equals negative log left paren 6 right paren minus log left paren 10 to the power of negative 5 right paren; hence pH equals negative 0.8 minus left paren negative 5 right paren equals 4.2. Option A is incorrect because pH equals 4.0 for 1 times 10 to the power of negative 4 M HNO subscript 3 left paren aq right paren. Option C is incorrect because pH equals 5.0 for 1 times 10 to the power of negative 5 M HNO subscript 3 left paren aq right paren. Option D is incorrect because pH equals 6.0 for 1 times 10 to the power of negative 6 M HNO subscript 3 left paren aq right paren.
Domain IV—Science Learning, Instruction and Assessment
Competency 021—The teacher understands research-based theoretical and practical knowledge about teaching science, how students learn science and the role of scientific inquiry in science instruction.
37. Which of the following is an element of inquiry-based science instruction?
- A teacher-led question-and-answer session
- A video presentation of science principles to be included in a unit of study
- A student forming a hypothesis prior to a lab activity
- A student writing a report after researching information on the Internet
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because inquiry-based learning does involve students proposing a hypothesis prior to designing an experiment to test the hypothesis. Option A is incorrect because a teacher asking questions is important, but it is not an element of inquiry-based science instruction. Option B is incorrect because videos can be helpful, but they are not elements of inquiry-based learning. Option D is incorrect because writing reports can have value, but it is not an element of inquiry-based learning.
38. Which of the following student responses is an example of correct conceptual understanding?
- Air has no mass
- The Sun is a star
- Heavy objects cannot float
- The Moon and the Sun are the same size
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because the Sun is a star. Option A is incorrect because air does have mass. Option C is incorrect because heavy objects can float. Option D is incorrect because the Sun is much larger than the Moon.
Competency 022—The teacher knows how to monitor and assess science learning in laboratory, field and classroom settings.
39. Which of the following is a type of summative assessment?
- A final examination
- A homework exercise
- An interview
- A question-and-answer session
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because it occurs after completion of learning and assesses what has been learned and how well it has been learned. Option B is incorrect because it is a type of formative assessment. Option C is incorrect because it is a type of diagnostic assessment. Option D is incorrect because it is a type of formative assessment.
40. Giving a short quiz before starting a new unit is most appropriate to help with which of the following?
- Planning the major content outcomes for the unit
- Discovering what prior knowledge or misconceptions the students may have
- Assessing which students will probably learn the most from the upcoming unit
- Assessing which students will require more of the teacher's time outside of class
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because a brief quiz can reveal the areas of prior knowledge as well as any misconceptions the students may have that should be addressed during the unit. Option A is incorrect because planning the major content outcomes should have already been done. Option C is incorrect because a short quiz cannot predict which students will learn the most from the unit. Option D is incorrect because a short quiz is not adequate to assess how much help various students will need outside of class.